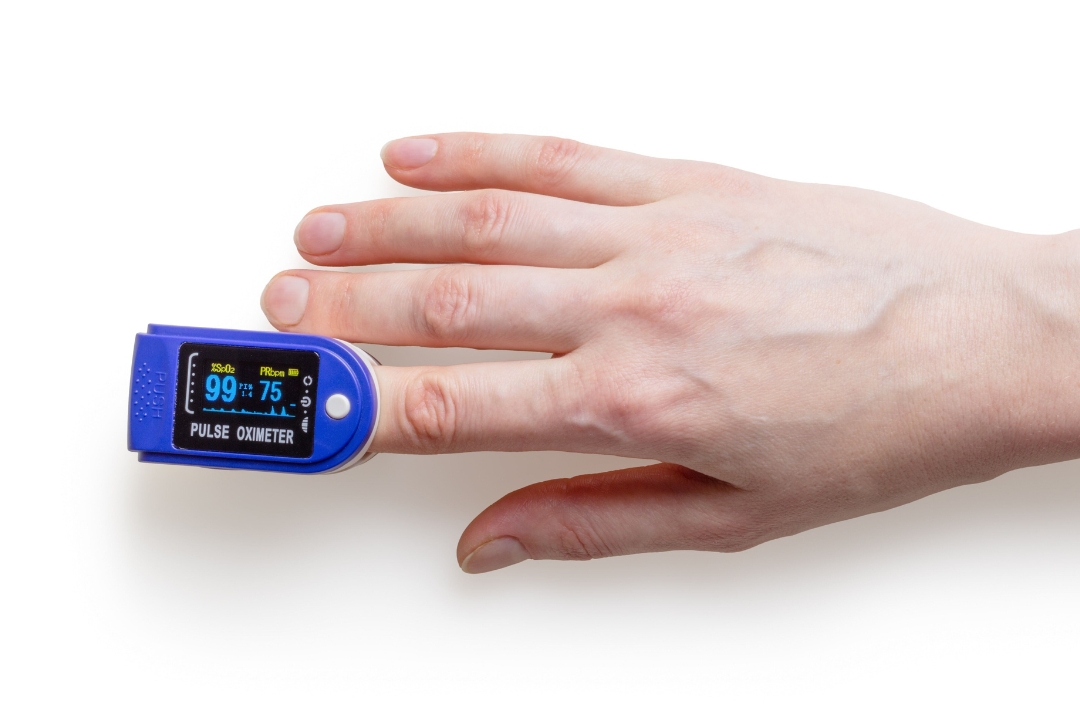
Medical devices play a vital role in modern healthcare, aiding in the diagnosis, treatment, and management of various medical conditions. Pacemakers that carefully monitor the electrical signals of the heart, imaging devices that detect cancerous cells, insulin pumps that stabilize blood glucose levels — such technological advancements have revolutionized healthcare. These innovations are truly lifesaving devices!
It’s in this context that pad printing plays an all-important role for medical devices. In this article, we’ll cover how manufacturers of medical devices can harness the power of pad printing while keeping in mind some of the pad printing challenges that come with regulations.
Importance of Warnings and Safety Instructions
When used on medical devices, pad printing provides crucial warnings, safety guidelines, and instructions about proper usage and maintenance procedures. By incorporating such warnings and instructions on medical devices, manufacturers are prioritizing patient and user safety, reducing the likelihood of accidents.
However, pad printing on medical devices is unlike pad printing on commercial items such as household goods — the text is often tiny for practical purposes instead of being design-oriented and eye-catching. But because they contain lifesaving instructions, it is incredibly important that the small text on medical devices is clearly legible. Here’s a scenario to illustrate the challenges and importance of applying safety instructions with pad printing:
HealthFirst — a medical device manufacturer — is responsible for producing disposable syringes which are essential for administering medications and vaccinations. HealthFirst needs to provide markings that indicate the volume of medication within the syringe. They would also need to include safety instructions such as “Handle with Care.”
The challenge of pad printing safety instructions on medical devices is that these instructions would need to be precise and clearly legible, and they’d need to remain that way. It’s very important that these markings and instructions do not rub off, wash off, or fade.
Essential Identification for Inventory Management
Pad printing also serves the purpose of providing essential identification on medical devices. The identification can take the form of serial numbers, batch numbers, or other unique codes.
These types of identification help healthcare providers keep track of items running low on stock and/or missing items. Additionally, identification makes it easy to discern the origins of the medical device such as brand, model, and year it was made. If a certain batch of medical devices was problematic, it could be traced back to the manufacturing company so they could resolve the issue.
Imagine if a medical device had a printed code, but upon examining it, it was difficult to see if one of the numbers was a 1 or a 7. Thinking that it was a 7, the healthcare worker input code into their system incorrectly, causing errors in inventory management. Identification codes need to be precise and readable.
The Challenges of Regulatory Compliance
Image source: https://www.freepik.com/free-vector/iso-certification-stamp-collection_10360578.htm
In the United States, inks printed on medical devices must meet FDA standards, which include:
- Biocompatibility: There’s a chance that the ink on the medical device comes in contact with the patient’s body. The manufacturer of the device needs to ensure that if this happens, the patient doesn’t endure any adverse reactions as a result of this contact.
- Material compatibility: The ink used for pad printing should be compatible with the medical device. The ink should not compromise the device’s performance or safety.
- Cleanliness and sterilization: Cleanliness is important for preventing the spread of disease and infections in healthcare settings. Contaminated medical equipment can harbor harmful microorganisms, so equipment needs to be sterilized often. It’s important that the ink on medical devices does not interfere with sterilization routines.
- Audits and inspections: Regulation authorities often conduct audits or inspections to make sure that the medical device complies with regulations.
In order to meet regulatory compliances, pad printing for medical devices is often done in ISO-certified clean rooms. However, it is not sufficient for the medical device to be manufactured in an ISO-certified clean room alone; the ink itself also needs to be ISO-certified.
Marabu Inks for Medical Products
Marabu takes great pride in offering a solution that caters to the unique needs of the medical device industry. With a deep understanding of the critical requirements involved in pad printing for medical devices, we have engineered a specialized ink product that fulfills the following:
- Biocompatible: Our ink is formulated using biocompatible materials to protect patients. Your medical devices remain safe throughout their ongoing use.
- Durability: We understand that medical devices go through a lot. That’s why our ink is engineered to withstand the challenges of the healthcare environment such as heavy usage, wear and tear, and sterilization routines.
- Precision printing: When it comes to pad printing for medical devices, achieving perfection is key for safeguarding patient and user safety and for meeting regulatory compliance. Our ink guarantees high precision, which means you can count on consistently sharp and accurate markings and identifiers.
- ISO-certified: Our inks are ISO-certified and have been since 1995. We guarantee that your medical devices comply with ISO-certification ink requirements.
As part of our service, you have the option to send your substrate to us (in this case, your medical device), and we’ll test our inks on that substrate. We’ll even provide technical support in case you have any questions. While shopping our inks for medical devices, you can also find more printing tips here. Ready to start on your pad printing job? Contact your printing partners at Marabu today.